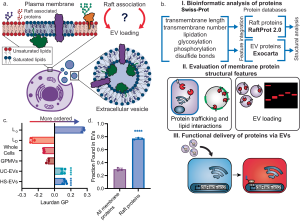
In a preclinical experimental study, Obuchi et al. (2025)—with contributions from the Department of Neurosurgery at Leiden University Medical Center—engineered extracellular vesicles for selective cargo loading and enhanced functional delivery, using a modified CD63 scaffold and VSV-G fusion, with in vivo validation in mouse brain models. 1)
🚫 1. Rebranding Complexity as Innovation The authors tout a modular EV engineering system using CD63, mCherry, FLAG-tags, nanobody fusions, and VSV-G. But this is not scientific ingenuity—it’s molecular bricolage. Each component is repurposed from older literature and glued together without real conceptual novelty. The result? A bloated acronym soup with more moving parts than scientific value.
⚠️ What’s pitched as a breakthrough is closer to a tech demo in search of a clinical rationale.
❌ 2. Absence of Disease-Relevant Application Despite name-dropping CRISPR, Cre, and Cas9, no disease context is addressed. No glioma model. No neurodegenerative target. No proof that the cargo accomplishes anything biologically meaningful in the recipient tissue. The mouse brain “validation” is just a fluorescent readout, not a therapeutic outcome.
The cargo arrives, but so what? This is payload delivery without a payload purpose.
🧪 3. Methodological Blind Spots No quantification of EV heterogeneity or functional subpopulations.
No rigorous comparison with alternative delivery systems (e.g., AAVs, lipid nanoparticles).
No evidence of endosomal escape for actual cytoplasmic/nuclear action.
No dose-response curves, toxicity profiling, or repeatability metrics.
This is a biotech prototype, not a therapy-in-the-making.
🔥 4. VSV-G: The Short-Term High, Long-Term Problem The use of VSV-G, a viral fusogen with broad tropism and high immunogenicity, is particularly careless. While it boosts in vitro uptake and helps “sell” delivery efficiency, it introduces a critical translational liability: poor specificity, potential immune activation, and unsuitability for clinical use.
❝Putting VSV-G on EVs is like installing a rocket engine on a paper boat—it moves faster, but it’s doomed to burn out or sink.❞
🧱 5. Structural Inefficiency and Complexity The system requires:
Engineering CD63 with dual tags
Fusing cargo to a nanobody
FLAG-based purification
VSV-G pseudotyping
TEV protease cleavage
This is logistically unscalable for clinical or industrial production and riddled with points of failure. The more components you bolt on, the more it resembles a lab curiosity, not a deliverable platform.
📉 6. Journal Inflation and Institutional Complacency The publication in J Extracell Vesicles is not a mark of impact, but rather a reflection of how EV journals have drifted into translational cosplay, applauding synthetic elegance over clinical relevance. The heavyweight affiliations (MGH, Harvard, etc.) likely ensured acceptance despite the absence of therapeutic depth or mechanistic rigor.
🧠 Conclusion: A study more interested in showing what’s technically possible than what’s biologically meaningful. It trades therapeutic relevance for engineering flair, while ignoring the hard questions of targeting, safety, scalability, and necessity.
This is not a step toward Evidence-based medicine—it’s a flashy side road to nowhere.