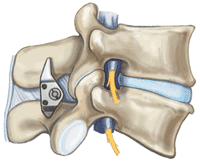
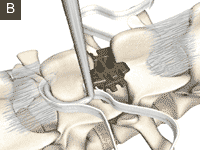
Interspinous spacer
J.Sales-Llopis
Neurosurgery Department, University General Hospital of Alicante, Foundation for the Promotion of Health and Biomedical Research in the Valencian Region (FISABIO), Alicante, Spain
Group of spinal implants used to treat lumbar spinal degenerative disease.
This kind of device is classified as part of the group of the dynamic stabilization systems of the spine.
A large number of interspinous devices (ID) have been introduced to the lumbar spine market as an alternative to conventional decompressive surgery in managing symptomatic lumbar spinal pathology, especially in the older population. Despite the fact that they are composed of a wide range of different materials including titanium, polyetheretherketone, and elastomeric compounds, the aim of these devices is to unload the lumbar facet joint, restore foraminal height, lower intradisk pressure, and provide motion-preserving stabilization.
Indications
Systematic Reviews
A systematic literature review of interspinous dynamic stabilization, including DIAM, Wallis, Coflex, and X-STOP, was conducted to assess its safety and efficacy.
The search was done in Korean and English, by using eight domestic databases which included KoreaMed and international databases, such as Ovid Medline, Embase, and the Cochrane Library. A total of 306 articles were identified, but the animal studies, preclinical studies, and studies that reported the same results were excluded. As a result, a total of 286 articles were excluded and the remaining 20 were included in the final assessment. Two assessors independently extracted data from these articles using predetermined selection criteria. Qualities of the articles included were assessed using Scottish Intercollegiate Guidelines Network (SIGN).
The complication rate of interspinous dynamic stabilization has been reported to be 0% to 32.3% in 3- to 41-month follow-up studies. The complication rate of combined interspinous dynamic stabilization and decompression treatment (32.3%) was greater than that of decompression alone (6.5%), but no complication that significantly affected treatment results was found. Interspinous dynamic stabilization produced slightly better clinical outcomes than conservative treatments for spinal stenosis. Good outcomes were also obtained in single-group studies. No significant difference in treatment outcomes was found, and the studies compared interspinous dynamic stabilization with decompression or fusion alone.
No particular problem was found regarding the safety of the technique. Its clinical outcomes were similar to those of conventional techniques, and no additional clinical advantage could be attributed to interspinous dynamic stabilization. However, few studies have been conducted on the long-term efficacy of interspinous dynamic stabilization. Thus, the authors suggest further clinical studies be conducted to validate the theoretical advantages and clinical efficacy of this technique 1).
Devices
Biomechanics
The primary biomechanical effect of the ID is reduced extension with associated reduced lumbar facet joint loads and smaller decrease in foraminal height.
The ID had little effect on sagittal instantaneous axis of rotation (IAR) or on motion or facet loads in other directions 2).
In extension, they overcompensate the instability caused by the defect and restricted extension to about 50% compared to the intact state. In contrast, in flexion, lateral bending, and axial rotation the values of the range of motion stayed similar compared to the defective state. Intradiscal pressure after implantation was similar to that of the intact specimens in flexion, lateral bending, and axial rotation but much smaller during extension; 50,000 load cycles increased the range of motion in all motion planes by no more than 20%, but in extension motion this was still less than in the intact state 3).
Coflex, Wallis, Diam and X-Stop interspinous implants had a similar effect on the flexibility: they strongly stabilized and reduced the intradiscal pressure in extension, but had almost no effect in flexion, lateral bending and axial rotation 4).
Results
The system with the lowest stiffness, DIAM, displayed nearly no stabilization of the treated segment, whereas the system with the highest stiffness, WALLIS and COFLEX, was most pronounced. This applies also for the correlation between device stiffness and ID. In flexion only the degree of stabilization is in correlation with the tensile stiffness, whereas the ID stays constant and is not affected by the different tensile stiffness. ID is not able to stabilize in the frontal and transversal plane. Furthermore ID does not substantially alter the location of the center of rotation (COR) 5).
Although patients may obtain some benefits from interspinous spacers implanted through a minimally invasive technique, interspinous spacer use is associated with a higher incidence of reoperation and higher cost. The indications, risks, and benefits of using an interspinous process device should be carefully considered before surgery 6).
Use of these implants has been very common but to date, no long-term clinical follow-up regarding clinical and radiological aspects are available. The high rate of reoperation, recurrence of symptoms and progression of degenerative changes is evident in the literature. If these devices are effectively a miracle cure for lumbar spinal stenosis, why do the utilization and implantation of IPD remain extremely controversial and should they be investigated further? Excluding the problems related to the high cost of the device, the main problem remains the pathological substrate on which the device is explicit in its action: the degenerative pathology of the spine 7).
Although the initial reports represented the IPD as a safe, effective, and minimally invasive surgical alternative for relief of neurological symptoms in patients with low back degenerative diseases, recent studies have demonstrated less impressive clinical results and higher rate of failure than initially reported 8).
The key points to ensure surgical effect and to reduce non-device-related complications are mastering surgical indications and thorough intra-operative decompression 9).
Twelve-month reoperation rates and index hospitalization costs were significantly higher among patients who underwent ID compared with laminectomy for lumbar spinal stenosis 10).
Early failure was significantly more common in patients treated with an ID for spinal stenosis and lumbar deformity, whereas reoperation due to symptomatic adjacent segment pathology was most common in patients treated with laminectomy and fusion. Laminectomy alone had the highest rate of survival.Level of Evidence: 3 11).
A double blinded study could not confirm the hypothesized short term advantage of interspinous process device over conventional “simple” decompression and even showed a fairly high reoperation rate after interspinous process device implantation 12).
The ID may be a suitable device to provide immediate flexion-extension balance after a unilateral laminotomy, but the bilateral pedicle screw system (BPSS) provides greater immediate stability in lateral bending and axial rotation motions. Both PLIF constructs performed equivalently in flexion-extension and axial rotation, but the PLIF-BPSS construct is more resistant to lateral bending motions. Further biomechanical and clinical evidence is required to strongly support the recommendation of a stand-alone interspinous fusion device or as supplemental fixation to expandable posterior interbody cages 13).
At the involved level, the hypermobility of the lumbar spinous processes (LSP) was seen in degenerative disc disease (DDD) and hypomobility of the LSP in degenerative spondylolisthesis (DLS) patients. The data may be instrumental for improving ID surgeries that are aimed at reducing post-operative complications such as bony fracture and device dislocations 14).
Complications
The majority of previously published studies are based on data with only shortterm follow-up or small patient numbers. In particular, the results of ID for the treatment of different indications have not been evaluated separately. Complications and long-term results still need to be established 15).
Heterotopic Ossification around a dynamic interspinous device may hamper motion preservation, and heterotopic ossification is a potential mid- and long-term complication 16).
Case series
2017
Long-term Clinical Outcomes and Quality of Life in Elderly Patients Treated with Interspinous Devices for Lumbar Spinal Stenosis in the Department of Neurosurgery, Catholic University of Rome, Rome, Italy. 17).