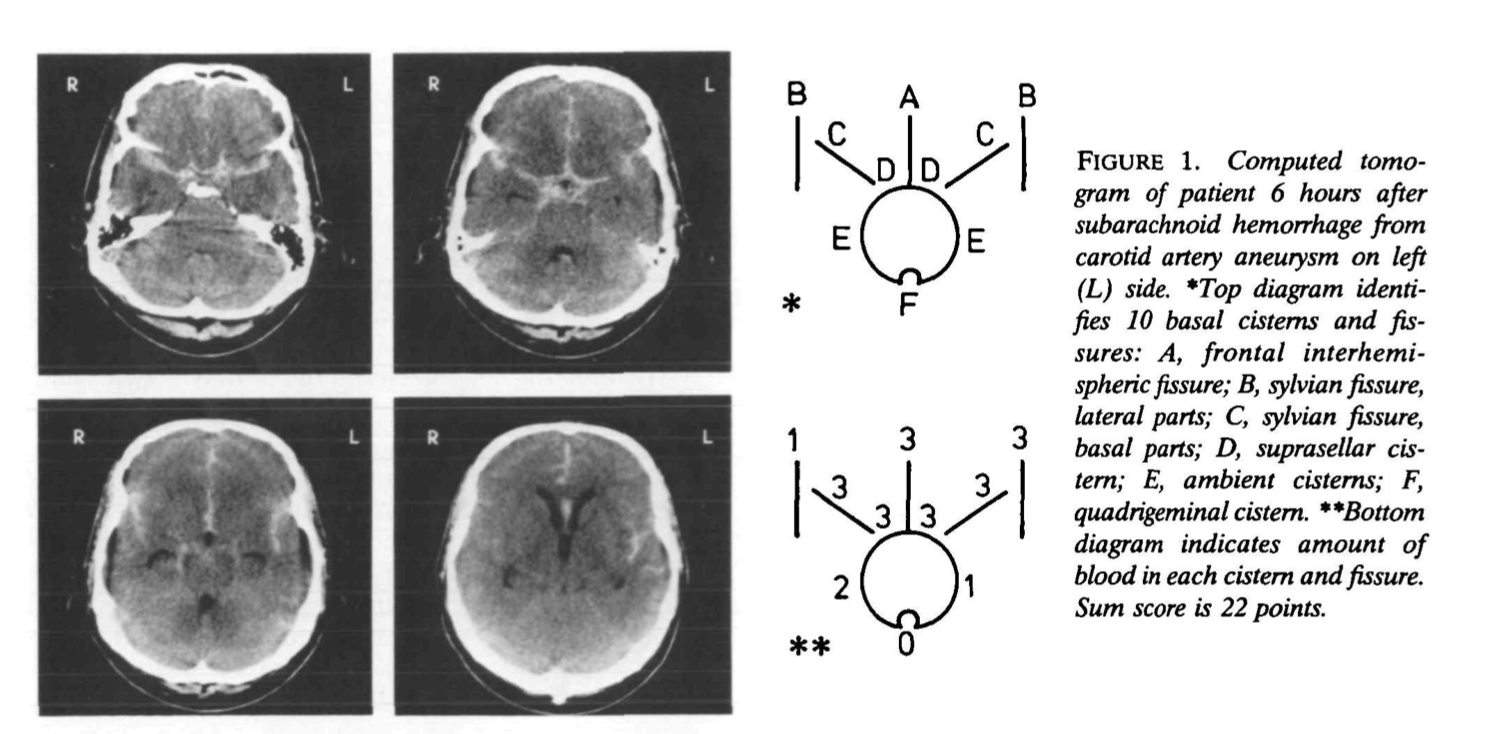
Hijdra sum score
Each of 10 basal cisterns and fissures is graded separately on a semiquantitative scale, according to the amount of extravasated blood: 0, no blood; 1, a small amount of blood; 2, moderately filled with blood; or 3, completely filled with blood. The density of the clot was not considered. Clots that had expanded the original size of a cistern or fissure were still graded as 3. The total amount of subarachnoid hemorrhage (sum score) was calculated by adding the 10 scores and ranged from 0 to 30. When an occasional cistern or fissure was considered to be inadequately visualized, they interpolated by assigning to that cistern or fissure the average score of the others.
Figure 1 shows a CT scan and a corresponding diagram as an example of the grading system.
The grading scale for the amount of blood in the four ventricles was constructed in a comparable fashion, as follows: 0, no blood; 1, sedimentation of blood in the posterior part; 2, partly filled with blood; or 3, completely filled with blood. The total amount of intraventricular blood (sum score) was the total of the four scores and ranged from 0 to 12 1)
Initial radiographic severity of aneurysmal subarachnoid hemorrhage aSAH was independently associated with the occurrence of different complications during aSAH and its final outcome. The Hijdra sum score showed the highest diagnostic accuracy and robust predictive value for early detection of the risk of DCI, in-hospital mortality, and unfavorable outcome after aSAH 2)
Yuan et al. compared automated blood volumes against traditional markers of bleeding (modified Fisher score [mFS], Hijdra sum score [HSS]) in 190 SAH patients for prediction of vasospasm, DCI, and functional status (mRS) at hospital discharge.
Results: Combined cisternal and sulcal volume was better correlated with mFS and HSS than cisternal volume alone (ρ=0.63 vs. 0.58 and 0.75 vs. 0.70, p<0.001). Only blood volume in combined cisternal plus sulcal compartments was independently associated with DCI (OR 1.023 per ml, 95% CI 1.002-1.048), after adjusting for clinical factors while ventricular blood volume was not. Total and specifically sulcal blood volume was strongly associated with poor outcome (OR 1.03 per ml, 1.01-1.06, p=0.006 and OR 1.04, 1.00-1.08 - for sulcal) as was HSS (OR 1.06 per point, 1.00-1.12, p=0.04), while mFS was not (p=0.24).
An automated imaging algorithm can measure the volume of bleeding after SAH within individual compartments, demonstrating cisternal plus sulcal (and not ventricular) blood contributes to the risk of DCI/vasospasm. Automated blood volume was independently associated with outcome, while qualitative grading was not. 3).
The following three factors were shown to predict acute hydrocephalus transiency and therefore included in the DOTAHAS score, ranging from 0 to 7 points: Hunt and Hess grade ≥ 3 (1 point), modified Fisher grade 4 (2 points), and Ventricular Hijdra Sum Score (vHSS) ≥ 6 (4 points). Patients scoring ≥ 3 points had a significantly higher risk for EVD (P < 0.0001) than other patients. The newly developed DOTAHAS score can be useful in identifying patients with transient acute hydrocephalus. Further score evaluation is needed. 4).
A retrospective analysis was performed upon patients prospectively included in the registry of SAH patients between July 2015 to April 2020. The amount of cisternal and intraventricular blood were assessed semi-quantitatively on acute and subacute CT scans performed after early resuscitation. A clot clearance rate was calculated from their comparison. The primary endpoint was the occurrence of a DCI. A total of 349 patients were included in the study; 80 (22.9%) experienced DCI. In those patients, higher Fisher grades were observed on acute (p = 0.026) and subacute (p = 0.003) CT scans. On the subacute CT scan, patients who experienced DCI had a higher amount of blood, either at the cisternal (median Hijdra sum score: 11 vs 5, p < 0.001) or intraventricular (median Graeb score: 4 vs 2, p < 0.001) level. There was a negative linear relationship between the cisternal clot clearance rate and the risk of DCI. The assessment of the amount of subarachnoid blood and clot clearance following resuscitation after aneurysmal SAH can be useful for the prediction of neurological outcome 5).
7: Das KK, Singh S, Bhaisora KS, Jaiswal AK, Behari S. Letter: Utility of the Hijdra Sum Score in Predicting Risk of Aneurysm in Patients With Subarachnoid Hemorrhage: A Single-Center Experience With 550 Patients. Neurosurgery. 2020 Oct 15;87(5):E604. doi: 10.1093/neuros/nyaa341. PMID: 32735662.
8: Kole MJ, Simard JM. In Reply: Utility of the Hijdra Sum Score in Predicting Risk of Aneurysm in Patients With Subarachnoid Hemorrhage: A Single-Center Experience With 550 Patients. Neurosurgery. 2020 Oct 15;87(5):E605. doi: 10.1093/neuros/nyaa342. PMID: 32735656.
Kole et al. conducted a retrospective study of all patients aged ≥18 yr admitted to a single center from March 2008 to March 2018 with nontraumatic SAH (n = 550). Patient information was compared between those with and without aneurysm to identify potential predictors. Odds ratios obtained from a logistic regression model were converted into scores which were summed and tested for predictive ability.
Results: Female sex, higher modified Fisher or Hijdra score, nonperimesencephalic location, presence of intracerebral hemorrhage, World Federation of Neurosurgical Societies (WFNS) score ≥3, need for cerebrospinal fluid diversion on admission, and history of tobacco use were all entered into the multivariable analysis. Greater modified Fisher, greater Hijdra score, WFNS ≥3, and hydrocephalus present on admission were significantly associated with the presence of an aneurysm. A model based on the Hijdra score and SAH location was generated and validated.
Conclusion: We show for the first time that the Hijdra score, in addition to other factors, may assist in identifying patients at risk for an aneurysm on cerebrovascular imaging. A simple scoring tool based on patient sex, SAH location, and SAH burden can assist in predicting the presence of an aneurysm in patients with nontraumatic SAH. 6).
The Fisher scale, modified Fisher scale, and Hijdra sum score are all associated with clinical DCI. The risk of DCI, however, does not increase with increasing Fisher grade as opposed to the modified Fisher scale. Furthermore, the modified Fisher scale was more commonly significantly associated with DCI than the Fisher scale, which may advocate using the modified Fisher in future SAH-related studies 7).
The aim of a cohort study was to investigate the prognostic value of the Hijdra sum scoring system for the functional outcome in patients with Fisher grade 3 aSAH, in order to improve the risk stratification within this Fisher category.
Methods: Initial CT scans of 72 prospectively enrolled patients with Fisher grade 3 aSAH were analyzed, and cisternal, ventricular, and the total amount of blood were graded according to the Hijdra scale. Additionally, space-occupying subarachnoid blood clots were assessed. The outcome was evaluated after 6 months.
Results: Within the subgroup of Fisher grade 3, aSAH patients with an unfavorable outcome showed a significantly larger cisternal Hijdra sum score (HSS: 21.1 ± 5.2) than patients with a favorable outcome (HSS: 17.6 ± 5.9; p = 0.009). However, both the amount of ventricular blood (p = 0.165) and space-occupying blood clots (p = 0.206) appeared to have no prognostic relevance. After adjusting for the patient's age, gender, tobacco use, clinical status at admission, and presence of intracerebral hemorrhage, the cisternal and total HSS remained the only independent parameters included in multivariate logistic regression models to predict functional outcome (p < 0.01).
Conclusion: The cisternal Hijdra score is fairly easy to perform and the present study indicates that it has an additional predictive value for the functional outcome within the Fisher 3 category. We suggest that the Hijdra scale is a practically useful prognostic instrument for risk evaluation after aSAH and should be applied more often in the clinical setting. 8).
For Dupont et al. Hijdra sum score and a history of smoking are the strongest predictors of cerebral vasospasm on angiography. HSS is superior to the MFS as a radiologic grading tool to predict the occurrence of angiographic vasospasm after aneurysmal subarachnoid hemorrhage 9) They proposed that only CT scans obtained within 24 h of a subarachnoid bleeding event should be used to estimate the risk of vasospasm 10).
Norden et al. investigated the interobserver variation of the Hijdra and Fisher scales for the amount of extravasated blood and the predictive values of these scales for delayed cerebral ischemia and outcome.
Methods: For 132 patients admitted within 48 hours after SAH three observers assessed the amount of blood on the initial CT scan by means of the Hijdra and Fisher scale. We analyzed interobserver agreement with kappa statistics and used multivariate logistic regression for the association with delayed cerebral ischemia and clinical outcome.
Results: The interobserver agreement of all three pairs of observers was good for the Hijdra scale (kappas for total sum scores ranging from 0.67 to 0.75) and mild to moderate for the Fisher scale (kappas ranging from 0.37 to 0.55). For the Hijdra scale the risk of DCI was higher for intermediate (OR 4.2; 95% CI 1.1-16.3) and large (OR 3.6; 95% CI 0.8-16.4) amounts of blood with small amount as reference. Fisher grade III (OR 1.0; 95% CI 0.2-5.2) and IV (OR 0.3; 95% CI 0.02-4.0) were not related with DCI. For the Hijdra scale and clinical outcome we found an increasing risk for poor outcome with intermediate (OR 3.9; 95% CI 1.0-15.9) and large (OR 10.7; 95% CI 2.3-50.1) amounts of blood. Such a relation was not found for Fisher grade III (OR 1.2; 95% CI 0.2-7.0) and IV (OR 0.2; 95% CI 0.01-3.4).
Conclusions: For the Hijdra scale we found a distinct better interobserver agreement than for the Fisher score. Moreover, the Hijdra scale was an independent prognosticator for DCI and clinical outcome, which was not the case for the Fisher score.: van 11)