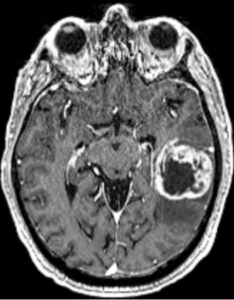
In a retrospective observational cohort study, Zerbib et al., from the Department of Radiation Oncology, Institut Universitaire du Cancer de Toulouse Oncopole (IUCT-Oncopole), Claudius Regaud; INSERM UMR 1037, Cancer Research Center of Toulouse (CRCT); IRT Saint-Exupéry; Department of Engineering and Medical Physics, IUCT-Oncopole; Biostatistics & Health Data Science Unit, IUCT-Oncopole; Department of Neuroradiology, Hôpital Pierre-Paul Riquet, CHU Purpan; Department of Medical Oncology & Clinical Research Unit, IUCT-Oncopole; Pathology and Cytology Department, CHU Toulouse, IUCT-Oncopole; CerCo, Université de Toulouse, CNRS, UPS, CHU Purpan; Department of Neurosurgery, Hôpital Pierre-Paul Riquet, CHU Purpan; and University Toulouse III – Paul Sabatier, published in The Oncologist, sought to evaluate and compare the clinical outcomes of patients with molecular glioblastoma (molGB) and histological glioblastoma (histGB) treated with standard radio-chemotherapy. They also assessed whether artificial intelligence (AI) models could accurately distinguish molGB without contrast enhancement (CE) from low-grade gliomas (LGG) using MRI FLAIR imaging features.
Conclusion: Patients with molGB and histGB showed similar overall survival under standard treatment.
-
However, molGB without contrast enhancement (CE) demonstrated a significantly better median overall survival (31.2 vs 18 months).
-
AI models based on FLAIR MRI features were able to differentiate non-enhancing molGB from LGG, achieving a best-performing ROC AUC of 0.85.
→ These findings support the clinical relevance of non-enhancing molGB as a distinct subgroup with better prognosis and highlight the potential diagnostic utility of AI tools in radiologically ambiguous cases.
This study presents itself as cutting-edge — mixing radiotherapy outcomes with artificial intelligence — but beneath the polished language and deep learning jargon lies a set of predictable flaws:
❶ Retrospective and underpowered: A 132-patient cohort — already heterogeneous — is further subdivided into histGB, molGB with CE, and molGB without CE. Statistical comparisons across these small subgroups are unreliable.
❷ The AI angle? Yes, a deep learning model differentiates molGB without CE from LGG with a ROC AUC of 0.85. Impressive? Only until you realize there is no external validation, no real-world deployment, and no error analysis. As usual, AI serves as a fashionable add-on — not a clinically deployable tool.
❸ Overstated survival difference: That non-enhancing molGB patients live longer (31.2 vs 18 months) is intriguing — but remains unexplained. There is no analysis of methylation subclasses, no mention of MGMT promoter methylation, and no adjustment for diagnostic or therapeutic delays. Are these findings rooted in biology or bias? The question isn’t asked.
❹ Radiologic ambiguity ignored: Although the paper acknowledges that molGB can mimic LGG radiologically, it fails to address the clinical consequence: these tumors may be underdiagnosed and undertreated. AI is referenced, but no clinical workflow is proposed to resolve this problem.
❺ Conclusion inflation: The abstract promises “diagnostic utility.” In reality, it delivers a prototype model with unclear application. Once again, deep learning is praised, but no clinician can use it — not now, not soon.
In short: a visually attractive study, full of fashionable buzzwords and polite omissions.
Good for citations, weak for clinical change.
More radiogenomic theater than paradigm shift.Zerbib C, Robinet L, Ken S, Cavillon A, Roques M, Larrieu D, Siegfried A, Roux FE, Berjaoui A, Cohen-Jonathan Moyal E. Clinical outcome and deep learning imaging characteristics of patients treated by radio-chemotherapy for a “molecular” glioblastoma. Oncologist. 2025 Jun 4;30(6):oyaf127. doi: 10.1093/oncolo/oyaf127. PMID: 40542584.