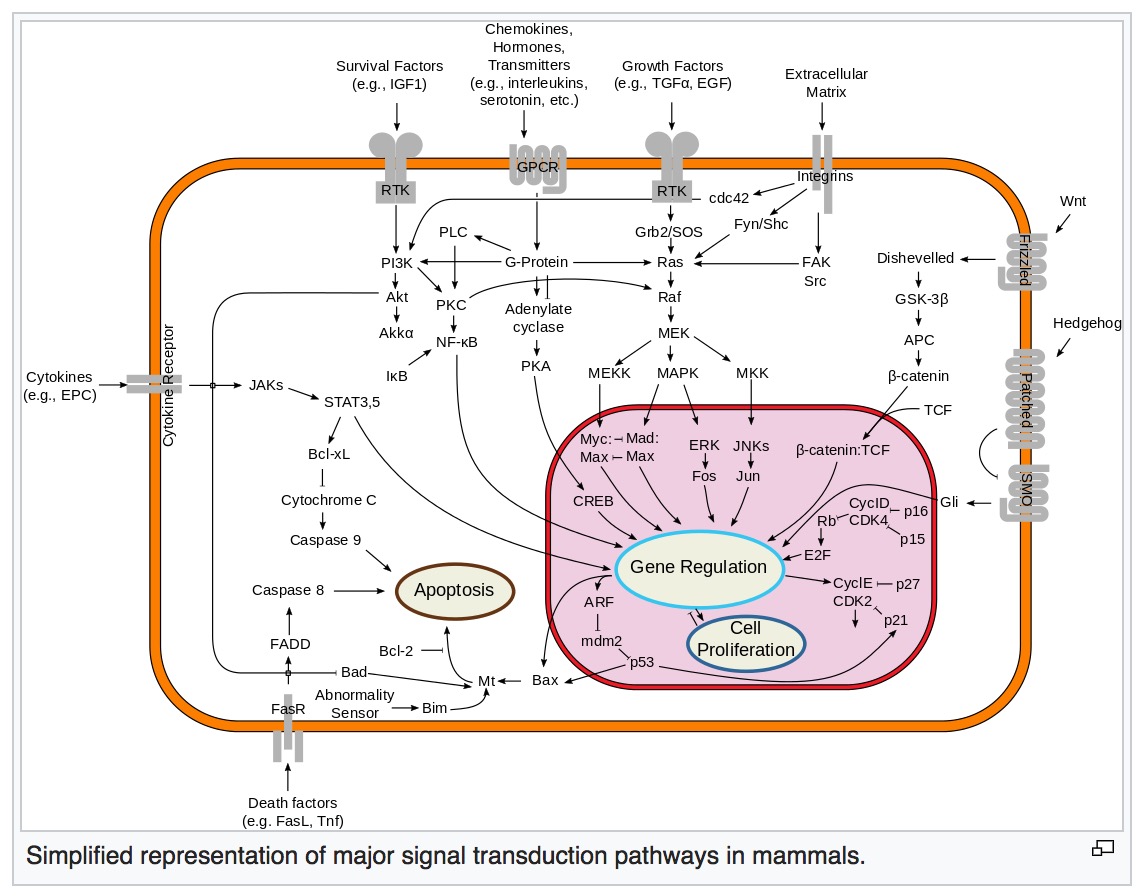
Wnt signaling pathway
The Wnt signaling pathways are a group of signal transduction pathways that begin with proteins that pass signals into a cell through cell surface receptors. The name Wnt is a portmanteau created from the names Wingless and Int-1.
Using Caenorhabditis elegans as a model, Shi et al. uncover that a Wnt signaling pathway in the gut regulates synaptic development in the brain. A canonical Wnt signaling pathway promotes synapse formation through regulating the expression of the neuropeptides encoding gene nlp-40 in the gut, which functions through the neuronally expressed GPCR/AEX-2 receptor during development. Wnt-NLP-40-AEX-2 signaling likely acts to modulate neuronal activity. The study revealed a genetic role of the gut in synaptogenesis and identifies a novel contribution of the gut-brain axis 1)
Aberrant regulation of the Wnt signaling pathway plays an important role in tumorigenesis.
It plays many essential roles in the regulation of the progenitor cell fate, developmental decisions, proliferation during embryonic development, and adult tissue homeostasis.
In a paper, Majidinia et al., briefly introduce Wnt/β-catenin signaling pathway and discuss how it integrally contributes to both stem and cancer stem cell maintenance. Finally, they summarize the current understanding of the role of Wnt/β-catenin signaling in the development and regeneration of heart, lung, liver, bone, and cartilage 2).
Wnt signaling pathways regulate proliferation, motility and survival in a variety of human cell types. DKK1 gene codes for a secreted Wnt inhibitory factor. It functions as tumour suppressor gene in breast cancer and as a pro-apoptotic factor in glioma cells.
Wnt/β-catenin and Hepatocyte Growth Factor (HGF)/c-Met signaling are hyperactive in human gliomas, where they regulate cell proliferation, migration and stem cell behavior.
Wnt/β-catenin signaling pathway is frequently dysregulated in human tumors and plays a critical role in tumorigenesis; however, the roles of microRNAs in mediating Wnt/β-catenin pathway are not well understood.
Expression of WNT3a, cytoplasmic β-catenin and TCF4 was significantly associated with the histological malignancy grade and with a worse prognosis for patients with glioma 3).