The Response Assessment in Neuro-Oncology Criteria for high-grade Glioma
The RANO criteria is a set of guidelines used for assessing the response to neuro-oncology treatment, particularly for high-grade gliomas.
The RANO criteria help standardize the evaluation of treatment response in clinical trials and clinical practice. They take into account various imaging modalities, clinical status, and sometimes histopathological findings.
Key aspects
Imaging Modalities:
MRI (Magnetic Resonance Imaging): RANO criteria heavily rely on MRI to evaluate changes in tumor size, enhancement patterns, and edema.
Perfusion and Diffusion Imaging: Advanced imaging techniques may be considered for a more comprehensive assessment of treatment response.
Response Categories:
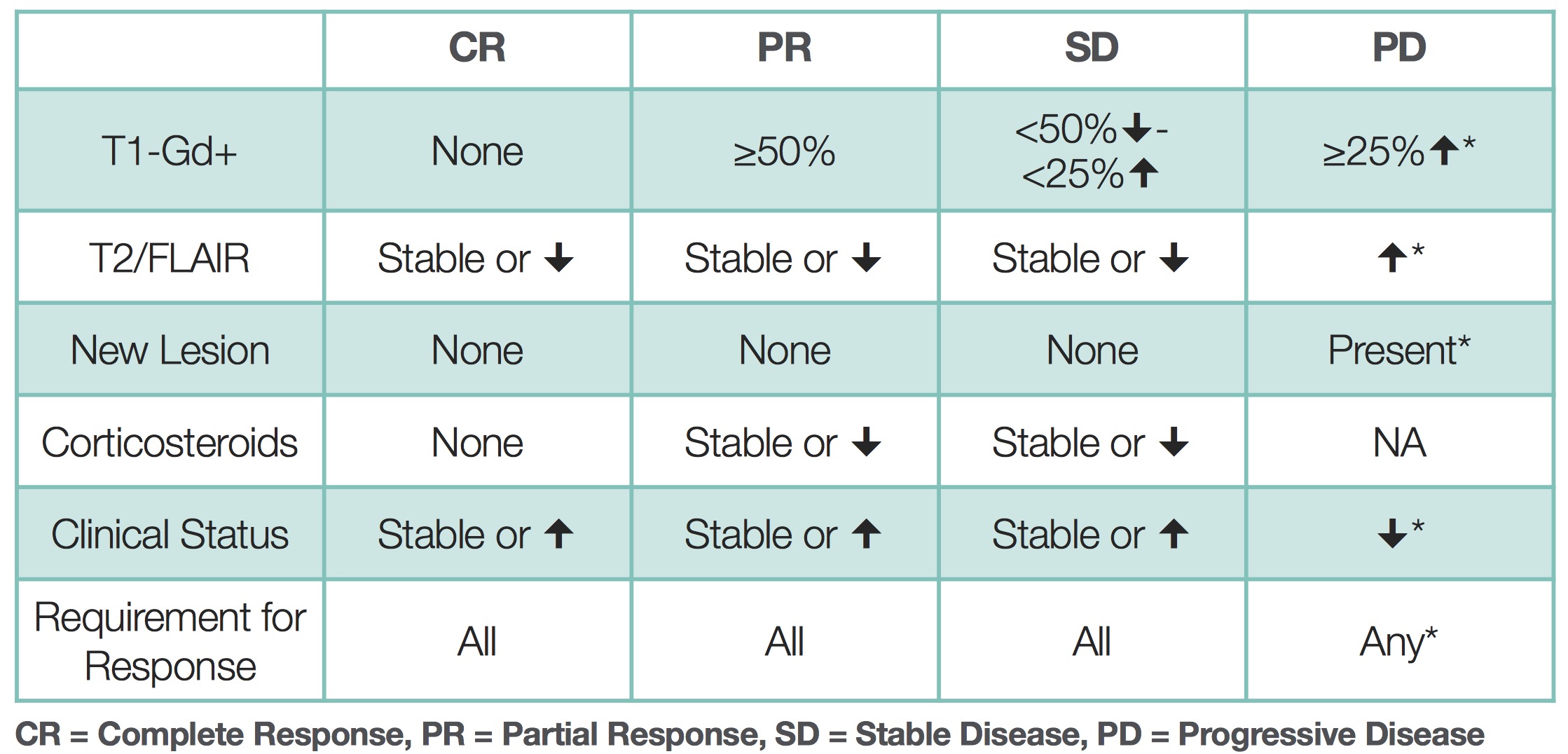
Complete Response (CR): Disappearance of all enhancing tumors on consecutive scans, stable or decreasing corticosteroid dose, and stable or improved clinical status.
Partial Response (PR): Significant decrease in the size of enhancing tumor (>50%), stable or decreasing corticosteroid dose, and stable or improved clinical status.
Stable Disease (SD): Not meeting criteria for CR, PR, or progressive disease. Stable or decreased enhancing tumor size and stable corticosteroid dose.
Progressive Disease (PD): Increase in the size of the enhancing tumor, any new lesions, clinical deterioration, or increased corticosteroid dose.
Assessment Timing:
Response assessment is typically performed at regular intervals during and after treatment.
The first assessment often occurs within a few weeks after the start of treatment.
Clinical Assessment:
Clinical status, including neurological examination and corticosteroid use, is considered in addition to imaging findings.
Pseudoprogression and Treatment-Related Changes:
Recognition of pseudoprogression (temporary increase in enhancement related to treatment) and treatment-related changes is important to avoid misinterpretation of response.
Recist Criteria for Progressive Disease:
For patients with significant non-enhancing disease, the RANO criteria recommend incorporating the Response Evaluation Criteria in Solid Tumors (RECIST) criteria for assessing progression.
It's important to note that the RANO criteria are periodically updated to incorporate advancements in imaging technologies and treatment modalities. Additionally, the criteria may vary for different types of brain tumors and clinical trial protocols.
Sharma et al. summarized the RANO guidelines in patients with high-grade glioma, highlighting the key clinical and imaging criteria used for RANO evaluation and introducing the role of newer imaging and biomarkers 1).
The challenge to accurately determine brain tumor response by MRI both in daily practice and in clinical trials has led to the introduction of updated guidelines by the Response Assessment in Neuro-Oncology (RANO) working group 2).
Improved radiographic interpretation criteria, such as the Response Assessment in Neurooncology criteria, incorporate non-enhancing disease but still fall short of definitely distinguishing tumor progression, pseudoresponse, radionecrosis and pseudoprogression.
Acknowledging the limitations of using measurements of the enhancing component as the sole criteria for tumor response, the Response Assessment in Neuro-Oncology(RANO) Working Group has proposed new standardized response criteria for clinical trials in brain tumors.
The Response Assessment in Neurooncology Criteria (RANO), was published in 2010 3) , and are used to assess response to first-line treatment of glioblastoma, (as well as lower grade astrocytoma) 4) and have largely superseded the older Macdonald criteria (which only dealt with GBM) 5).
These new criteria take into account some factors, including change in the size of the T1 gadolinium-enhancing disease, change in T2/FLAIR signal abnormality, presence of new lesions, corticosteroid use, and change in clinical status, thus acknowledging our increased understanding of the complexity of brain tumors and treatment. These changes also underscore the limitations of assessment methods based solely on morphologic imaging and highlight the need for functional imaging techniques capable of assessing tumor tissue microstructure, metabolism, and physiology.
The RANO criteria, roughly similar to other systems, divides response into 4 types of response based on imaging (MRI) and clinical features 6) 7).
Leao et al. described relevant practical issues when evaluating patients with glioma, such as the need for imaging in the first 48 hours, the radiation therapy planning and isodose curves, the significance of T2/FLAIR hyperintense lesions, the impact of the timing for the evaluation after radiation therapy, and the definition of progressive disease on the histologic specimen. We also illustrate the correlation between the findings of conventional MR imaging with advanced techniques, such as perfusion, diffusion-weighted imaging, spectroscopy, and amino acid PET. Because many of the new lesions represent a mixture of tumor cells and tissue with radiation injury, the radiologist aims to identify the predominant component of the lesion and categorize the findings according to Response Assessment in Neuro-Oncology criteria so that the patient can receive the best treatment 8).
The Response Assessment in Neuro-Oncology (RANO) criteria for high-grade gliomas (RANO-HGG) and low-grade gliomas (RANO-LGG) were developed to improve the reliability of response assessment in glioma trials. Over time, some limitations of these criteria were identified, and challenges emerged regarding integrating features of the modified RANO criteria (mRANO) or the immunotherapy RANO (iRANO) criteria.
Informed by data from studies evaluating the different criteria, updates to the RANO criteria are proposed (RANO 2.0).
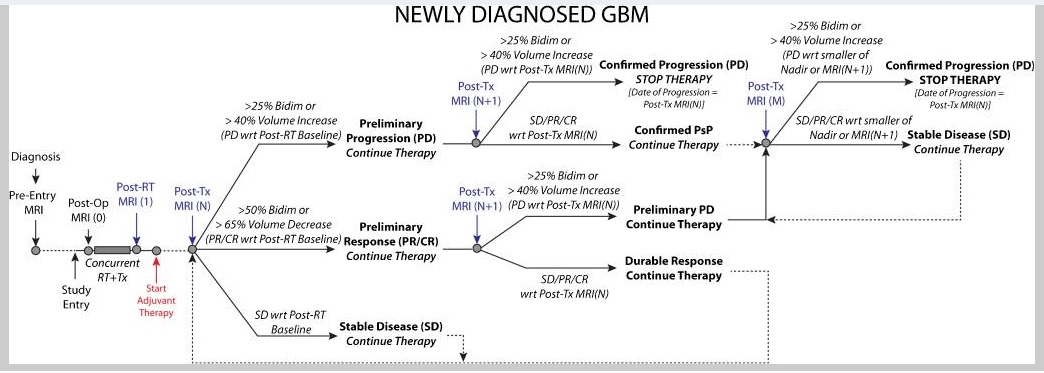
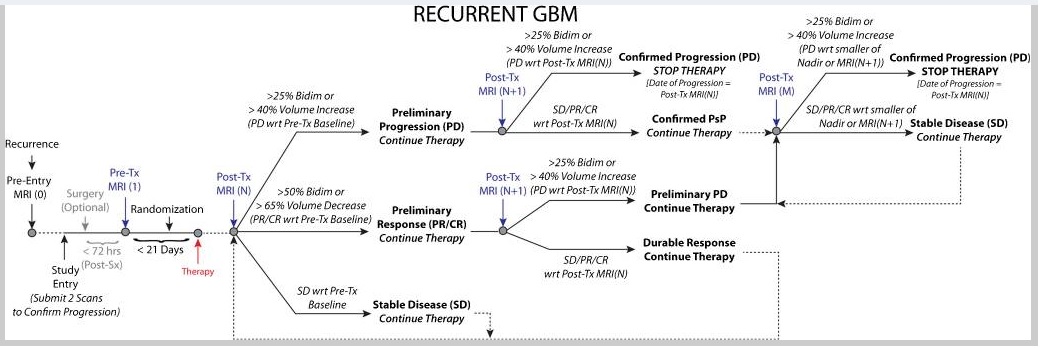
They recommend a standard set of criteria for both high- and low-grade gliomas, to be used for all trials regardless of the treatment modalities being evaluated. In the newly diagnosed setting, the postradiotherapy magnetic resonance imaging (MRI), rather than the postsurgical MRI, will be used as the baseline for comparison with subsequent scans. Since the incidence of pseudoprogression is high in the 12 weeks after radiotherapy, continuation of treatment and confirmation of progression during this period with a repeat MRI, or histopathologic evidence of unequivocal recurrent tumor, are required to define tumor progression. However, confirmation scans are not mandatory after this period nor for the evaluation of treatment for recurrent tumors. For treatments with a high likelihood of pseudoprogression, mandatory confirmation of progression with a repeat MRI is highly recommended. The primary measurement remains the maximum cross-sectional area of the tumor (two-dimensional) but volumetric measurements are an option. For IDH wild-type glioblastoma, the non-enhancing disease will no longer be evaluated except when assessing response to antiangiogenic agents. In IDH-mutated tumors with a significant non-enhancing component, clinical trials may require evaluating both the enhancing and non-enhancing tumor components for response assessment 9).
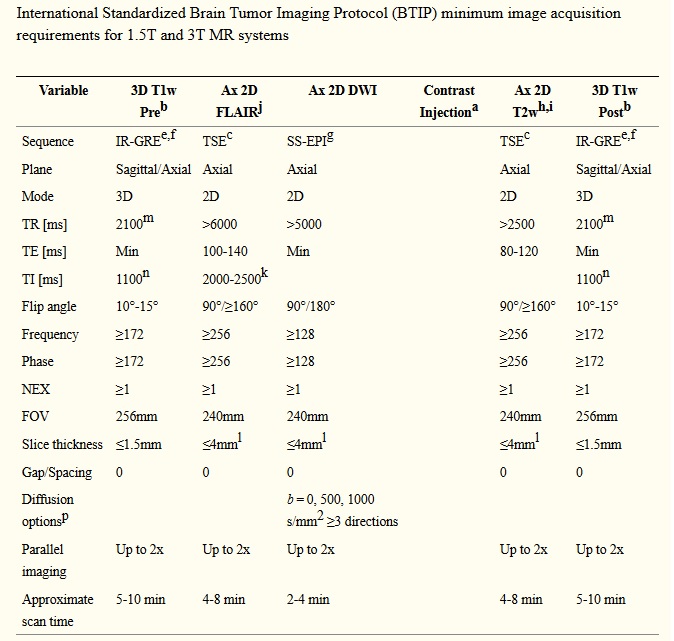
In response to a need for better standardization of image acquisition in GBM clinical trials 10) , a consensus paper was published outlining an “international brain tumor imaging protocol (BTIP)” with recommended sequences and parameters 11). At the core of this recommended protocol is parameter matched, pre- and post-contrast 3D (volumetric) inversion recovery gradient recalled echo (IR-GRE) images with less than 1.5-mm isotropic resolution, which allows for both bidimensional and volumetric measurements of enhancing tumor. When possible, this protocol should be employed for prospective clinical trials.
Ax = Axial; ADC = apparent diffusion coefficient; FLAIR = fluid attenuated inversion recovery; DWI = diffusion-weighted imaging; 3D = three dimensional; TSE = turbo spin echo; EPI = echo planar imaging; SS-EPI = single-shot echo planar imaging; GE-EPI = gradient echo echo planar imaging; 2DFL = two-dimensional FLASH (fast low angle shot) gradient recalled echo; MPRAGE = magnetization prepared rapid gradient-echo; A/P = anterior to posterior; R/L = right to left; NEX = number of excitations or averages; FOV = field of view; TE = echo time; TR = repetition time; TI = inversion time; PD = proton density; DSC = dynamic susceptibility contrast; IR-GRE = inversion-recovery gradient-recalled echo
a0.1 mmol/kg dose injection with a Gadolinium chelated contrast agent. Use of a power injector is desirable at an injection rate of 3-5cc/s
bPost-contrast 3D T1-weighted images should be collected with equivalent parameters to pre-contrast 3D T1-weighted images
cTSE = turbo spin echo (Siemens & Philips) is equivalent to FSE (fast spin echo; GE, Hitachi, Toshiba)
dFL2D = two-dimensional fast low angle shot (FLASH; Siemens) is equivalent to the spoil gradient recalled echo (SPGR; GE) or T1- fast field echo (FFE; Philips), fast field echo (FastFE; Toshiba), or the radiofrequency spoiled steady state acquisition rewound gradient echo (RSSG; Hitachi). A fast gradient echo sequence without inversion preparation is desired
eIR-GRE = inversion-recovery gradient-recalled echo sequence is equivalent to MPRAGE = magnetization prepared rapid gradient-echo (Siemens & Hitachi) and the inversion recovery spoiled gradient-echo (IR-SPGR or Fast SPGR with inversion activated or BRAVO; GE), 3D turbo field echo (TFE; Philips), or 3D fast field echo (3D Fast FE; Toshiba)
fA 3D acquisition without inversion preparation will result in different contrast compared with MPRAGE or another IR-prepped 3D T1-weighted sequences and therefore should be avoided
gIn the event of significant patient motion, a radial acquisition scheme may be used (e.g. BLADE [Siemens], PROPELLER [GE], MultiVane [Philips], RADAR [Hitachi], or JET [Toshiba]); however, this acquisition scheme is can cause significant differences in ADC quantification and therefore should be used only if EPI is not an option. Further, this type of acquisition takes considerably more time
hDual echo PD/T2 TSE is optional for possible quantification of tissue T2. For this sequence, the PD echo is recommended to have a TE < 25ms
iAdvanced sequences can be substituted into this time slot, so long as 3D post-contrast T1-weighted images are collected between 4 and 8 min after contrast injection
j3D FLAIR is an optional alternative to 2D FLAIR, with sequence parameters as follows per EORTC guidelines: 3D TSE/FSE acquisition; TE = 90-140ms; TR = 6000-10000ms; TI = 2000-2500ms (chosen based on vendor recommendations for optimized protocol and field strength); GRAPPA ≤ 2; Fat Saturation; Slice thickness ≤ 1.5mm; Orientation Sagittal or Axial; FOV ≤ 250 mm x 250 mm; Matrix ≥ 244×244
kChoice of TI should be chosen based on the magnetic field strength of the system (e.g. TI ≈ 2000ms for 1.5T and TI ≈ 2500ms for 3T)
lIn order to ensure comparable SNR older 1.5T MR systems can use contiguous (no interslice gap) images with 5mm slice thickness or increase NEX for slice thickness ≤4mm
nFor Siemens and Hitachi scanners. GE, Philips, and Toshiba scanners should use a TI = 400-450ms for similar contrast
mFor Siemens and Hitachi scanners. GE, Philips, and Toshiba scanners should use a TR = 5-15ms for similar contrast
pOlder model MR scanners that are not capable of >2 b-values should use b = 0 and 1000 s/mm2
If volumetric acquisition is not employed, or if retrospective evaluations of existing trial data are performed, then slice thickness plus interslice gap should be less than 5 mm. If the sum of the slice thickness and gap exceeds 5 mm, then slightly modified definitions of measurable disease should be used (e.g. measurable disease = largest perpendicular diameters > 2× slice thickness + gap).
Quantification of contrast enhancing tumor size or volume should be performed on contrast-enhanced T1-weighted digital subtraction maps in order to increase lesion conspicuity and better predict tumor burden in the presence of reduced vascular permeability as occurs during anti-angiogenic therapy 12) and/or T1 shortening from blood products or calcifications 13) 14). Further, the American College of Radiology (ACR) recommends this approach for identification and delineation of subtly enhancing bone and soft tissue lesions 15)).
Complete response
Disappearance of all enhancing measurable and non-measurable disease sustained for at least 4 weeks. The first scan exhibiting disappearance of all enhancing measurable and non-measurable disease is considered “preliminary CR”. If the second scan exhibits measurable enhancing disease with respect to the “preliminary CR” scan, then the response is not sustained, noted as pseudoresponse, PsR, and is now considered “preliminary PD” (note confirmed PD requires at least two sequential increases in tumor volume). If the second scan continues to exhibit disappearance of enhancing disease or emergence of non-measurable disease (less than 10mm bidimensional product), it is considered a durable CR and the patient should continue on therapy until confirmed PD is observed.
Patients must be off corticosteroids (or on physiologic replacement doses only).
Stable or improved clinical assessments (i.e. neurological examinations).
Note: Patients with non-measurable disease only at baseline cannot have CR; the best response possible is stable disease (SD).
Partial response
≥50% decrease in sum of products of perpendicular diameters or ≥65% decrease in total volume [54, 70, 96] of all measurable enhancing lesions compared with baseline, sustained for at least 4 weeks. The first scan exhibiting ≥50% decrease in sum of products of perpendicular diameters or ≥65% decrease in total volume [54, 70, 96] of all measurable enhancing lesions compared with baseline is considered “preliminary PR”. If the second scan exhibits PD with respect to the “preliminary PR” scan, then the response is not sustained, noted as pseudoresponse, PsR, and is now considered “preliminary PD” (note confirmed PD requires at least two sequential increases in tumor volume). If the second scan exhibits SD, PR, or CR, it is considered a durable PR and the patient should continue on therapy until confirmed PD is observed.
Steroid dose should be the same or lower compared with baseline scan.
Stable or improved clinical assessments.
Note: Patients with non-measurable disease only at baseline cannot have PR; the best response possible is stable disease (SD).
Stable Disease (SD)
Requires all of the following:
Does not qualify for CR, PR, or PD as defined above. Note this also applies to patients that demonstrate PsR when the confirmation scan does not show PD or PsP when the confirmation scan does not show PR/CR.
In the event that corticosteroid dose was increased (for new symptoms/signs) without confirmation of disease progression on neuroimaging, and subsequent follow-up imaging shows that the steroid increase was required because of disease progression, the last scan considered to show stable disease will be the scan obtained when the corticosteroid dose was equivalent to the baseline dose.
Progressive Disease (PD)
At least two sequential scans separated by at ≥4 weeks both exhibiting ≥25% increase in sum of products of perpendicular diameters or ≥40% increase in total volume [54, 70, 96] of enhancing lesions. The first scan exhibiting ≥25% increase in sum of products of perpendicular diameters or ≥40% increase in total volume [54, 70, 96] of enhancing lesions should be compared to the smallest tumor measurement obtained either at baseline (if no decrease) or best response (on stable or increasing steroid dose) and is noted as “preliminary PD.” If the second scan at least 4 weeks later exhibits a subsequent ≥25% increase in sum of products of perpendicular diameters or ≥40% increase in total volume of enhancing lesions relative to the “preliminary PD” scan, it is considered “confirmed PD” and the patient should discontinue therapy. If the second scan at least 4 weeks later exhibits SD or PR/CR, this scan showing “preliminary PD” is noted as “pseudoprogression”, PsP, and the patient should continue on therapy until a second increase in tumor size relative to the PsP scan is observed. Note that any new measurable (>10mm x 10mm) enhancing lesions should not be immediately considered PD, but instead should be added to the sum of bidimensional products or total volume representing the entire enhancing tumor burden. In the case where the baseline or best response demonstrates no measurable enhancing disease (visible or not visible), then any new measurable (>10mm x 10mm) enhancing lesions are considered PD after confirmed by a subsequent scan ≥4 weeks exhibiting ≥25% increase in sum of products of perpendicular diameters or ≥40% increase in total volume of enhancing lesions [54, 70, 96] relative to the scan first illustrating new measurable disease. The first scan exhibiting new measurable disease is noted as “preliminary PD.” If the second scan at least 4 weeks later exhibits a subsequent ≥25% increase in sum of products of perpendicular diameters or ≥40% increase in total volume [54, 70, 96] of enhancing lesions relative to the “preliminary PD” scan it is considered “confirmed PD” and the patient should discontinue therapy. If the second scan at least 4 weeks later exhibits SD, CR, PR, or becomes non-measurable, this scan showing “preliminary PD” is noted as “pseudoprogression”, PsP, and the patient should continue on therapy until a second increase in tumor size relative to the “preliminary PD”, or PsP, scan is observed. Note that any new measurable (>10mm x 10mm) enhancing lesions on the subsequent scan following the preliminary PD scan should not be immediately considered confirmed PD, but instead should be added to the sum of bidimensional products or total volume representing the entire enhancing tumor burden. Clear clinical deterioration not attributable to other causes apart from tumor (e.g. seizures, medication adverse effects, therapy complications, stroke, infection) or attributable to changes in steroid dose. Failure to return for evaluation as a result of death or deteriorating condition.
Measurement
The RANO criteria were, at least in part, developed to address the issues faced when measuring some lesions on Macdonald criteria, particularly:
lesions with central necrosis
T2 component
As such lesions are defined as “measurable” and “non-measurable”
Measurable lesions
A measurable lesions is measured as follows:
either CT or MRI
contrast enhancing
clearly defined margins
visible on two or more axial slices
preferably <5mm thick with 0mm skip
maximal diameter and second perpendicular measurement
at least 10mm in size (if slice thickness <5mm)
2 times slice thickness (if slice thickness >5mm)
From ref 1: “In the event there are interslice gaps, this also needs to be considered in determining the size of measurable lesions at baseline.” -
do not measure cystic cavity
Non-measurable lesions
Non-measurable lesions are generally those that do not meet the criteria above. Additionally, and worthy of specific mention, is a cystic / necrotic tumour, or one with a surgical cavity. In such cases only a solid peripheral nodular component should be measured, provided it fulfills the above 'measurable' criteria.
Again, it is not difficult to think of numerous examples where defining a 'nodule' is difficult. It is therefore crucial that the base line scan is available as well as the axes of initial measurement in assessing response.
The measurements are obtained from axial post contrast T1 images. The maximal diameter is obtained, and then the second diameter is obtained at right angles to the first. The product of these measurements is then used for the purpose of comparison.
Case series
The purpose of a study was to evaluate RANO criteria in glioblastoma multiforme (GBM), with respect to the Macdonald criteria and changes in contrast enhancement (CE) volume. Related variations in relative cerebral blood volume (rCBV) were investigated.
Forty-three patients diagnosed between 2006 and 2010 were included. All underwent surgical resection, followed by temozolomide-based chemoradiation. MR images were retrospectively reviewed. Times to progression (TTPs) according to RANO criteria, Macdonald criteria and increased CE volume (CE-3D) were compared, and the percentage change in the 75th percentile of rCBV (rCBV75) was evaluated.
After a median follow-up of 22.7 months, a total of 39 patients had progressed according to RANO criteria, 32 according to CE-3D, and 42 according to Macdonald. Median TTPs were 6.4, 9.3, and 6.6 months, respectively. Overall agreement was 79.07% between RANO and CE-3D and 93.02% between RANO and Macdonald. The mean percentage change in rCBV75 at RANO progression onset was over 73% in 87.5% of patients.
In conclusion, the findings suggest that CE-3D criterion is not yet suitable to assess progression in routine clinical practice. Indeed, the accurate threshold is still not well defined. To date, in Tensaouti et al. opinion, early detection of disease progression by RANO combined with advanced MRI imaging techniques like MR perfusion imaging and diffusion remains the best way to assess disease progression. Further investigations that would examine the impact of treatment modifications after progression determined by different criteria on overall survival would be of great value 16).