Notch signaling pathway
The Notch signaling pathway is a highly conserved signaling pathway that plays a critical role in cell-to-cell communication during embryonic development and in maintaining tissue homeostasis in adults. The pathway is named after the Notch protein, a transmembrane receptor that is a key component of the pathway.
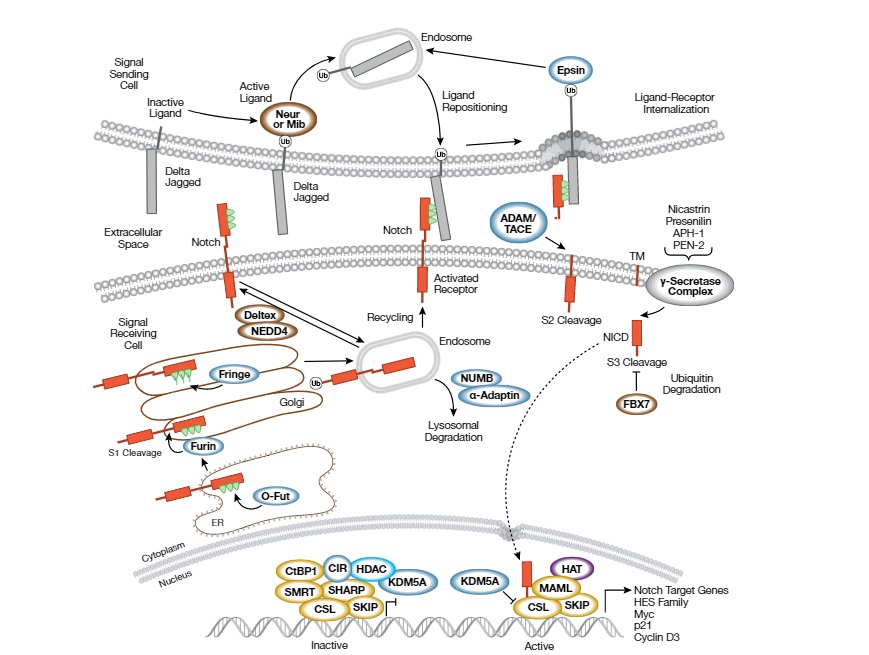
The Notch signaling pathway is activated when a transmembrane ligand, such as Jagged or Delta-like protein, on one cell binds to the extracellular domain of the Notch receptor on an adjacent cell. This binding triggers a series of proteolytic cleavages that release the intracellular domain of the Notch receptor, which then translocates to the nucleus and activates the transcription of target genes.
The target genes activated by the Notch pathway vary depending on the cell type and context. Notch signaling is involved in a variety of cellular processes, including cell fate determination, proliferation, differentiation, and apoptosis. Dysregulation of the Notch pathway has been implicated in many diseases, including cancer, autoimmune diseases, and developmental disorders.
The Notch pathway is a complex and tightly regulated pathway, and researchers are continuing to explore the many mechanisms involved in its activation and regulation. Understanding the Notch pathway and its associated signaling molecules may provide insights into the development of new therapies for a range of diseases.
The pathway is named after the Notch receptor, a transmembrane protein that is activated by binding to its ligands, such as Delta-like and Jagged proteins, on the surface of adjacent cells.
When a ligand binds to the Notch receptor, it undergoes a series of proteolytic cleavages that release the intracellular domain of the receptor (NICD). The NICD translocates to the nucleus where it binds to the transcription factor CSL (CBF1/RBPJk in mammals) and recruits coactivators to activate the transcription of target genes, including the Hes and Hey families of transcription factors.
The Notch pathway is involved in a variety of developmental processes, including neurogenesis, myogenesis, hematopoiesis, angiogenesis, and epithelial-to-mesenchymal transition. Dysregulation of the Notch pathway has been implicated in a number of human diseases, including cancer, cardiovascular diseases, and neurodegenerative disorders. Therefore, the Notch pathway is a promising target for the development of novel therapeutics.
Jagged1 (JAG1) is one of five cell surface proteins (ligands) that interact with 4 receptors in the mammalian Notch signaling pathway. The Notch Signaling Pathway is a highly conserved pathway that functions to establish and regulate cell fate decisions in many organ systems. Once the JAG1-NOTCH (receptor-ligand) interactions take place, a cascade of proteolytic cleavages is triggered resulting in activation of the transcription for downstream target genes.
Notch is present in all metazoans, and mammals possess four different notch receptors, referred to as NOTCH1, NOTCH2, NOTCH3, and NOTCH4. The notch receptor is a single-pass transmembrane receptor protein. It is a hetero-oligomer composed of a large extracellular portion, which associates in a calcium-dependent, non-covalent interaction with a smaller piece of the notch protein composed of a short extracellular region, a single transmembrane-pass, and a small intracellular region.
Notch signaling promotes proliferative signaling during neurogenesis, and its activity is inhibited by Numb to promote neural differentiation.
It is suggested to promote the development and maintenance of cerebral arteriovenous malformations (AVMs), and an increasing wall shear stress (WSS) contributes to AVM rupture. Little is known about whether WSS impacts Notch signaling, which is important for understanding the angiogenesis of AVMs. WSS was measured in arteriovenous fistulas (AVF) surgically created in 96 rats at different time points over a period of 84 days. The expression of Notch receptors 1 and 4 and their ligands, Delta1 and 4, Jagged1, and Notch downstream gene target Hes1 was quantified in “nidus” vessels. The interaction events between Notch receptors and their ligands were quantified using proximity ligation assay. There was a positive correlation between WSS and time (r = 0.97; P < 0.001). The expression of Notch receptors and their ligands was upregulated following AVF formation. There was a positive correlation between time and the number of interactions between Notch receptors and their ligands aftre AVF formation (r = 0.62, P < 0.05) and a positive correlation between WSS and the number of interactions between Notch receptors and their ligands (r = 0.87, P < 0.005). In conclusion, an increasing WSS may contribute to the angiogenesis of AVMs by activation of Notch signaling 1).
Notch is a cell-cell signaling pathway that is involved in a host of activities including development, oncogenesis, skeletal homeostasis, and much more.
The Notch signaling pathway is activated after Spinal Cord Injury, leading to hypomyelination in the dorsolateral prefrontal cortex (dlPFC), and DAPT can inhibit the Notch signaling pathway and improve mechanical and thermal hyperalgesia thresholds. These findings provide a new target for the treatment of neuropathic pain caused by SCI 2).
More specifically, research has demonstrated the importance of Notch signaling in osteogenic differentiation, bone healing, and the development of the skeleton. The craniofacial skeleton is complex and understanding its development has remained an important focus in biology. In a review, we briefly summarize what recent research has revealed about Notch signaling and the current understanding of how the skeleton, skull, and face develop. They then discuss the crucial role that Notch plays in both craniofacial development and the skeletal system, and what importance it may play in the future 3).
Oncolytic herpes simplex virus type 1 (oHSV) infection of brain tumors activates NOTCH. NOTCH induced immunosuppressive myeloid cell recruitment limited anti-tumor immunity. Translationally, these findings support the use of NOTCH inhibition in conjunction with oHSV therapy 4)
Dysregulation
Dysregulation of the Notch signaling pathway, which is highly conserved across species, can drive aberrant epigenetic modification, transcription, and translation. Defective gene regulation caused by dysregulated Notch signaling often affects networks controlling oncogenesis and tumor progression. Meanwhile, Notch signaling can modulate immune cells involved in anti- or pro-tumor responses and tumor immunogenicity. A comprehensive understanding of these processes can help with designing new drugs that target Notch signaling, thereby enhancing the effects of cancer immunotherapy. Li et al. provide an up-to-date and comprehensive overview of how Notch signaling intrinsically regulates immune cells and how alterations in Notch signaling in tumor cells or stromal cells extrinsically regulate immune responses in the tumor microenvironment (TME). They also discuss the potential role of Notch signaling in tumor immunity mediated by gut microbiota. Finally, they propose strategies for targeting Notch signaling in cancer immunotherapy. These include oncolytic virotherapy combined with inhibition of Notch signaling, nanoparticles (NPs) loaded with Notch signaling regulators to specifically target tumor-associated macrophages (TAMs) to repolarize their functions and remodel the TME, combining specific and efficient inhibitors or activators of Notch signaling with immune checkpoint blockers (ICBs) for synergistic anti-tumor therapy, and implementing a customized and effective synNotch circuit system to enhance safety of chimeric antigen receptor (CAR) immune cells 5).