Barricaid Annular Closing Device
The bulk of the information regarding the use of annular closure devices in the prevention of recurrent herniation following lumbar discectomy comes from a randomized clinical trial (RCT) to evaluate the use of Barricaid device (Barricaid, Intrinsic Therapeutics, Inc, Woburn, MA) 1).
Previously reported long-term outcomes (3 years) of this RCT have shown a significant decrease in symptomatic reherniation (14.8 vs 29.5%) and reoperation (11 vs 19.3%) rates when compared with the control group 2).
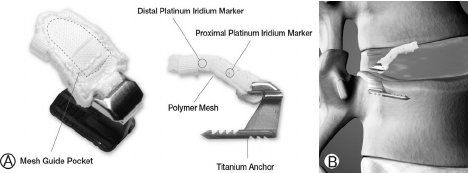
Barricaid is designed to close large defects in the annulus, so to prevent recurrent disc herniation, while allowing the surgeon to preserve more of the patient’s lumbar disc.
The Barricaid Anular Closure device consists of a woven polyester occlusion component intended to block an anular defect, while anchored to the adjacent vertebral body by a titanium bone anchor.
Reinforcing the annulus fibrosus with Barricaid during lumbar discectomy may slow the progression of facet joint degeneration 3).
The polymer mesh is placed on the inner surface of the disc annulus, using the disc pressure to help seal the defect against leakage of the nucleus pulposus. Once wound dissection and (partial, hemilaminectomy has been done, discectomy is followed. However, aggressive nucleus removal has been shown to result in significant back pain and worsened clinical outcomes. After discectomy, the annular defect size is measured and the appropriately sized device is chosen. The titanium anchor is inserted into the bone (parallel to the surface of the endplate) and the mesh forms a barrier that blocks the defect.
The device provides permanent fixation through the bone anchorage and remains inside the disc. During the procedure, fluoroscopic guidance is required to ensure the appropriate location of the device.
Annular closure device insertion allows more nucleus to be left inside of the annulus and restores intra-discal pressure. Because only partial volume is removed from the intervertebral disc, this procedure can preserve disc height and motion and reduce facet degeneration
Patients with primary lumbar disc herniation show Endplate changes (EPC) in the corresponding segments. There is a significant increase in lesion number and size within 12 months after discectomy. This increase is significantly more pronounced in the annular closure device (ACD) group. The presence and growth of EPC are not correlated with low-back pain or ODI 4).
Trials
Study registration: ClinicalTrials.gov (https://clinicaltrials.gov): NCT03986580 5)
Case series
Case reports
Discussion
Kurzbuch AR, Fournier JY, Tuleasca C. The annular closure device - panacea of lumbar disc herniation: how closed is closed enough for the intervertebral disc space? Acta Neurochir (Wien). 2021 Feb 19. doi: 10.1007/s00701-021-04764-9. Epub ahead of print. PMID: 33606100.