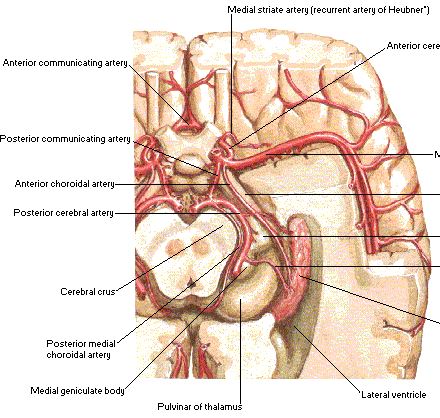
Anterior choroidal artery (AchA)
J.Sales-Llopis
Neurosurgery Department, University General Hospital of Alicante, Spain
Anatomically, the cisternal segment of the AChA originates from the posterolateral wall of the internal carotid artery (ICA), runs along the lateral recess of the basal cistern, attaches itself to the optic tract, which is follows to reach the crural cistern before entering the choroidal fissure.
Microdissection of 100 hemispheres from human cadavers were performed in order to study the anatomic characteristics of the anterior choroidal artery (AChA). One AChA per hemisphere was found. In 98% of hemispheres the AChA arose from the internal carotid artery (ACI) 2.4mm distal to the origin of the posterior communicating artery (ACoP) and 4.7mm proximal to the carotid bifurcation.
Perforating vessels
The available information about certain microanatomic features of the AChA perforators is incomplete. Precise knowledge of these vessels is necessary to understand the consequences of their occlusion and to safely operate in their region.
One or more perforating branches arose from communicating segment of ACI in 29% of hemispheres. The average calibre of the cisternal portion was 0.9mm and the plexal portion 0.7mm. The most frequent branches of the cisternal portion pass to the optic tract, cerebral peduncle, uncus and lateral geniculate body. Anastomosis were found between branches of the AChA and posterior cerebral artery, ACoP, middle cerebral artery and ACI 1).
The perforating branches ranged in number from 2 to 9 (mean, 4.6) and in diameter between 90 microm and 600 microm (mean, 317 microm). The most proximal perforator arose 3.2 mm on average caudal to the AChA origin. The most distal (capsulothalamic) perforator varied in size from 200 microm to 610 microm (mean, 431 microm). One or more of the perforators always originated from the AChA (100%), but some of them also from the uncal (33.3%) or parahippocampal branch (10%) of the AChA, either as individual vessels only (70%) or from common trunks (30%). The perforators gave off the peduncular (20%), optic (23.3%), or uncal side branches (26.7%).
The findings concerning the origin, position, number, size, branching, penetration site, and relationships of the AChA perforators gave the anatomic basis for safe operations in patients with AChA aneurysms or mediobasal limbic epilepsy 2).
Origin
An ectopic origin was observed in 4% of cases. The intracisternal segment of the anterior choroidal a. forms a neurovascular bundle with the optic tract and basal v.
Branches
Most of its intraparenchymatous branches arise from the cisternal segment, while branches supplying the optic tract, lateral geniculate body and thalamus arise from the intraplexual segment. Constant anastomoses exist with the vertebrobasilar system, specially the postero-lateral choroidal and posterior cerebral aa. 3).
A huge uncal branch of the anterior choroidal artery was found in one of 22 cerebral hemispheres. The uncal artery measured 0.7 mm in diameter. It gave off small branches to the uncus, as well as a large parahippocampal artery, the accessory anterior temporal artery, and the anterior hippocampal artery. This uncal artery supplied, in addition to the uncus, most of the ventromedial surface of the parahippocampal gyrus, part of its dorsal surface, the rostromedial portion of the lateral occipitotemporal gyrus, and the rostral part of the hippocampal formation. Such a huge uncal artery has implications for surgery in the uncohippocampal region 4).
Posterior communicating artery (★) and anterior choroidal artery (★★) too which are shown in the picture being retracted with a dissector.
The anterior choroidal artery (AChA) gave rise to an anterior uncal artery in 83% of hemispheres and a posterior uncal or unco-hippocampal artery in 98%. The plexal segment of the AChA gave off neural branches in 38%. The MCA was the site of origin of anterior uncal, unco-parahippocampal, or anterior parahippocampal arteries in 94% of hemispheres. An anterior uncal artery arose from the internal carotid artery (ICA) in 45% of hemispheres. The posterior cerebral artery (PCA) irrigated the entorhinal area through its anterior parahippocampal or hippocampo-parahippocampal branches in every case. A PCA bifurcation was identified in 89% of hemispheres, typically at the middle segment of the MTR. The most common pattern of bifurcation was by division into posteroinferior temporal and parieto-occipital arterial trunks. The anterior segment of the basal vein had a predominant anterior drainage in 35% of hemispheres, and the middle segment had a predominant inferior drainage in 16% 5).
An understanding of the vascular variability of the MTR is essential for accurate microsurgical resection of MTR AVMs.
Segments
Both parts of the cisternal segment of the AChA come into surgical view during surgeries for different pathologies in and around the perimesencephalic cisterns. However, attending to the artery's genu and defining pre- and postoptic parts during surgery may help the surgeon locate the origin and eventual course of these perforators, and even estimate the terminal areas of supply of most of the perforating arteries. The proposed classification system os Tanriover et al. can prove helpful in planning any operative procedure along the crural cistern and may reduce the probability of inadvertent injury to perforating branches of the cisternal segment.
The cisternal segment of the AChA was divided into pre- and postoptic parts that meet at the artery's genu, the most medial extension point of the cisternal segment where the artery makes an abrupt turn after passing under the optic tract. The preoptic part of the AChA extended from its origin at the inferomedial side of the internal carotid artery to the artery's genu, which is commonly located just inferomedial to the initial part of the optic tract. The postoptic part coursed within the crural cistern and extended from the genu to the inferior choroidal point. The genu of the AChA was 8 mm medial to the artery's origin and was located medial to the optic tract in 13% of the hemispheres. The postoptic part was longer than the preoptic part in all hemispheres and had more perforating arteries supplying critical deep structures (preoptic 3.4 per hemisphere vs postoptic 4.6 per hemisphere), and these results were statistically significant (p = 0.01). At the preoptic part, perforating arteries arose from the superolateral portion of the artery and coursed laterally; at the postoptic part, perforators arose from the inferomedial portion of the artery and coursed medially. Perforating arteries from both segments passed most commonly to the optic tract, followed by the anterior segment and apex of uncus in the preoptic part and the cerebral peduncle in the postoptic part. 6).
Structure
The anterior choroidal artery serves structures in the telencephalon, diencephalon, and mesencephalon:
choroid plexus of the lateral ventricle and third ventricle
optic chiasm and optic tract
internal capsule
lateral geniculate body
globus pallidus
tail of the caudate nucleus
hippocampus
amygdala
substantia nigra
red nucleus
crus cerebri
Clinical significance
The full extent of the damage caused by occlusion of the anterior choroidal artery is not known. However, studies show that the interruption of blood flow from this vessel can result in hemiplegia on the contralateral (opposite) side of the body, contralateral hemihypoesthesia, and homonymous hemianopsia. These symptoms are thought to arise from ischemic damage to the posterior limb of the internal capsule, thalamus, and optic chiasm/optic tract. However, the posterior limb of the internal capsule also receives lenticulostriate arteries from the Middle Cerebral Artery, thus creating partially redundant supply.
In malignant cerebral edema the involvement of the anterior choroidal artery can be subtle in the setting of a large infarct, but involvement of the uncus of the temporal lobe may lead to more rapid herniation 7).