In a retrospective observational cohort study, Zerbib et al., from the Department of Radiation Oncology, Institut Universitaire du Cancer de Toulouse Oncopole (IUCT-Oncopole), Claudius Regaud; INSERM UMR 1037, Cancer Research Center of Toulouse (CRCT); IRT Saint-Exupéry; Department of Engineering and Medical Physics, IUCT-Oncopole; Biostatistics & Health Data Science Unit, IUCT-Oncopole; Department of Neuroradiology, Hôpital Pierre-Paul Riquet, CHU Purpan; Department of Medical Oncology & Clinical Research Unit, IUCT-Oncopole; Pathology and Cytology Department, CHU Toulouse, IUCT-Oncopole; CerCo, Université de Toulouse, CNRS, UPS, CHU Purpan; Department of Neurosurgery, Hôpital Pierre-Paul Riquet, CHU Purpan; and University Toulouse III – Paul Sabatier, published in The Oncologist, sought to evaluate and compare the clinical outcomes of patients with molecular glioblastoma (molGB) and histological glioblastoma (histGB) treated with standard radio-chemotherapy. They also assessed whether artificial intelligence (AI) models could accurately distinguish molGB without contrast enhancement (CE) from low-grade gliomas (LGG) using MRI FLAIR imaging features.
Conclusion: Patients with molGB and histGB showed similar overall survival under standard treatment.
-
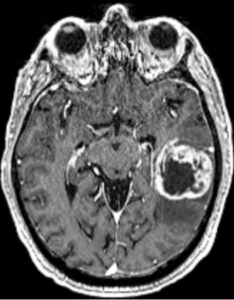
However, molGB without contrast enhancement (CE) demonstrated a significantly better median overall survival (31.2 vs 18 months).
-
AI models based on FLAIR MRI features were able to differentiate non-enhancing molGB from LGG, achieving a best-performing ROC AUC of 0.85.
→ These findings support the clinical relevance of non-enhancing molGB as a distinct subgroup with better prognosis and highlight the potential diagnostic utility of AI tools in radiologically ambiguous cases.
This study presents itself as cutting-edge — mixing radiotherapy outcomes with artificial intelligence — but beneath the polished language and deep learning jargon lies a set of predictable flaws: