In a Review Raziye Piranlioglu *et al.* from
| Affiliations | Harvey Cushing Neuro‑oncology Laboratories, Dept. Neurosurgery, Brigham and Women’s Hospital, Boston, MA, USA; Dana‑Farber Cancer Institute, Boston, MA, USA |
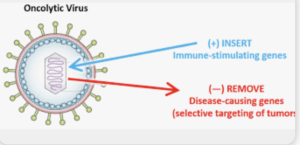
published in *Seminars in Immunology* with the Purpose to synthesize data from clinical trials of oncolytic viruses (OVs) in glioblastoma, evaluating immunomodulatory effects, delivery strategies, and challenges in assessing immune responses. They concluded that Oncolytic virus therapy is well tolerated in GBM trials and can convert the immunosuppressive microenvironment into an immunologically active state. However, limitations in post‑treatment sampling and delivery methods impede full understanding of biological mechanisms.
This review is a rehash of well‑known take‑home messages, offering little in the way of novel synthesis or incisive critique. The authors lean heavily on canonical trials (e.g., oHSV, adenovirus) but fail to integrate preclinical correlates from myeloid-targeting strategies, such as macrophage polarization dynamics or MDSC modulation. There’s no fresh mechanism, no meta‑analysis of response rates, and no exploration of why most trials remain phase I with limited impact. Sample‑scarcity is once again highlighted as a blocker—but no alternative trial designs (e.g., neoadjuvant window cohorts, liquid biopsy) are proposed. In short, the review scratches the surface of challenges without pushing the field forward.