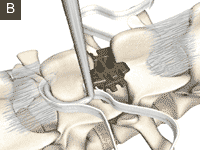
Wallis
The Wallis interspinous implant was invented in 1986 by Mr. Jacques Sénégas as one of the first modern interspinous devices. The primary intention of Sénégas was to improve segmental stabilisation, based on the unloading of the facet joints and the disc. The Wallis system is easy to handle and the early clinical results are promising. How far the early outcome will lead to good long-term results is unknown, because there are no long-term results in the literature till now. Limiting factors for the devices are the possible biomechanical overload, the loosening of the implants and the lytic zones around the spinous processes after more than 1 year 1).
Clinical effectiveness of the PEEK nonfusion spine implant Wallis (Abbott, Bordeaux, France; now Zimmer, Warsaw, IN) is well documented.
However, there is a lack of evidence on the long-term effects of this implant on bone, in particular its influence on structural changes of bone elements of the lumbar spine.
Experimental
Twenty-four male BB.4S rats aged 11 weeks underwent surgery for implantation of a PEEK nonfusion interspinous device or for a sham procedure in 3 groups of 8 animals each: (1) implantation at level L4-L5; (2) implantation at level L5-L6; and (3) sham surgery. Eleven weeks postoperatively osteolyses at the implant-bone interface were measured via radiograph, bone mineral density of vertebral bodies was analyzed using osteodensitometry, and bone mineral content as well as resorption of the spinous processes were examined by histomorphometry. RESULTS.: Resorption of the spinous processes at the site of the interspinous implant was found in all treated segments. There was no significant difference in either bone density of vertebral bodies or histomorphometric structure of the spinous processes between adjacent vertebral bodies, between treated and untreated segments and between groups.
These findings indicate that resorption of spinous processes because of a result of implant loosening, inhibit the targeted load redistribution through the PEEK nonfusion interspinous device in the lumbar spinal segment of the rat. This leads to reduced long-term stability of the implant in the animal model. These results suggest that PEEK nonfusion interspinous devices like the Wallis implants may have time-limited effects and should only be used for specified indications 2).