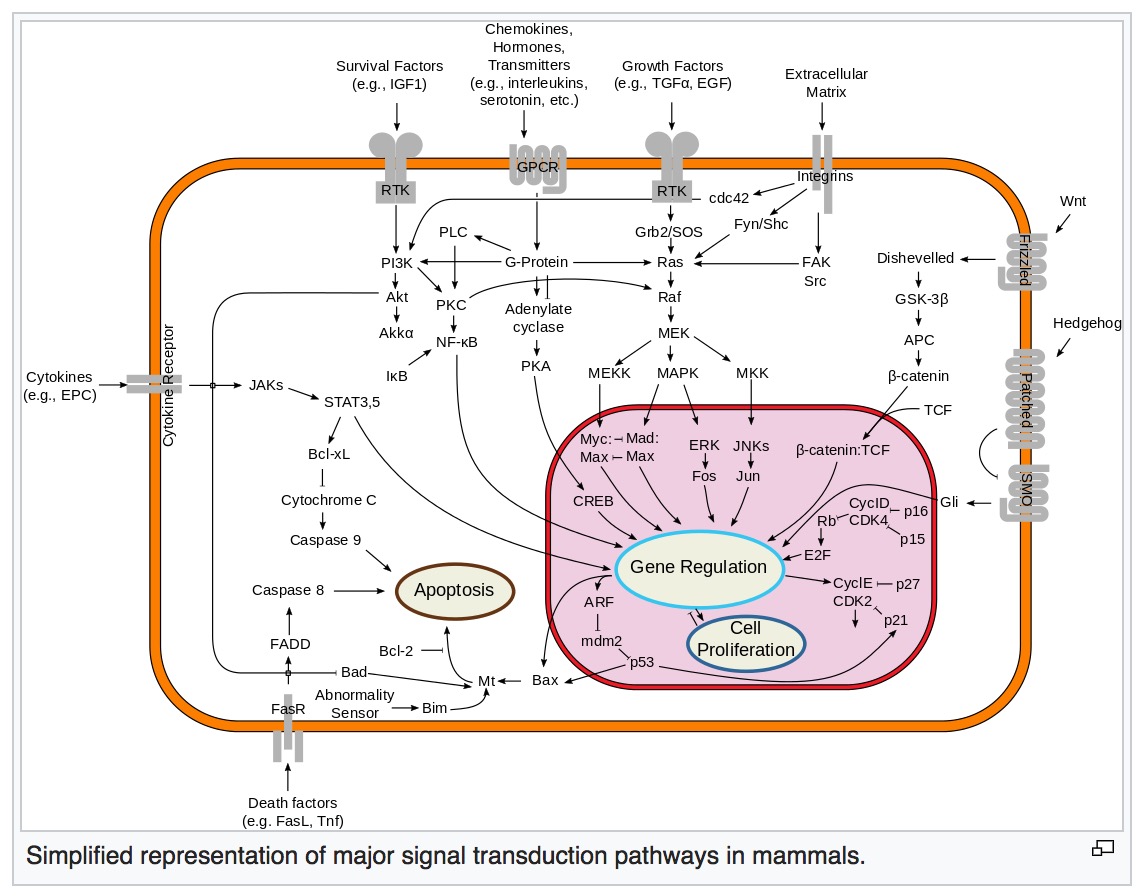
PI3K/AKT/mTOR pathway
The PI3K/AKT/mTOR pathway is an intracellular signaling pathway important in regulating the cell cycle. Therefore, it is directly related to cellular quiescence, proliferation, cancer, and longevity.
Dysregulation of the PI3K/Akt/mTOR signaling cascade has been associated with the pathology of neurodegenerative disorders, specifically Alzheimer's disease (AD).
While cancer metabolism in other organs is commonly associated with upregulated glycolysis (i.e. Warburg effect) and hyperactivation of PIK3/AKT/mTOR (PAM) pathways, the unique bioenergetic demands of the central nervous system may interact with these oncogenic processes to promote tumor progression in aging. Specifically, constitutive glycolysis and PIK3/AKT/mTOR signaling in glia may be dysregulated by age-dependent alterations in neurometabolic demands, ultimately contributing to pathological processes otherwise associated with PIK3/AKT/mTOR induction (e.g. cell cycle entry, impaired autophagy, dysregulated inflammation). Although several limitations to this theoretical model exist, the consideration of aberrant PIK3/AKT/mTOR signaling in glia during aging elucidates several therapeutic opportunities for brain tumors, including non-pharmacological interventions 1).
PI3K activation phosphorylates and activates AKT, localizing it in the plasma membrane.
AKT can have a number of downstream effects such as activating CREB, inhibiting p27, localizing FOXO in the cytoplasm, activating PtdIns-3ps, and activating mTOR which can affect transcription of p70 or 4EBP1.
There are many known factors that enhance the PI3K/AKT pathway including EGF, shh, IGF-1, insulin, and CaM.
The pathway is antagonized by various factors including PTEN, GSK3B, and HB9. In many cancers, this pathway is overactive, thus reducing apoptosis and allowing proliferation. This pathway is necessary, however, to promote growth and proliferation over differentiation of adult stem cells, neural stem cells specifically
It is the difficulty in finding an appropriate amount of proliferation versus differentiation that researchers are trying to determine in order to utilize this balance in the development of various therapies. Additionally, this pathway has been found to be a necessary component in neural long term potentiation.
The Phosphoinositide 3 kinase (PI3K)/Akt pathway is known to play a major role in angiogenesis. Studies have shown that the phosphatase and tensin homologue deleted on chromosome ten (PTEN), a tumor suppressor, is an antagonist regulator of the PI3K/Akt pathway and mediates angiogenesis by activating vascular endothelial growth factor (VEGF) expression.
PI3Ks are a family of related intracellular signal transducer enzymes capable of phosphorylating the 3 position hydroxyl group of the inositol ring of phosphatidylinositol (PtdIns).
The pathway, with oncogene PIK3CA and tumor suppressor PTEN, is implicated in insensitivity of cancer tumors to insulin and IGF1, in calorie restriction.
Current standard treatment for glioma patients is surgical removal followed by radiotherapy and adjuvant chemotherapy. Due to therapeutic resistance and tumor recurrence, efforts are ongoing to identify the molecules that are fundamental to regulate the tumor progression and provide additional methods for individual treatment of glioma patients. By studying the initiation and maintenance of glioma, studies focused on the targets of tyrosine kinase receptors including EGFR, PDGFR and other crucial signal pathways such as PI3K/AKT and RAS/RAF/MAPK pathway. Furthermore, recent advances in targeting immunotherapy and stem cell therapy also brought numerous strategies to glioma treatment 2).
PI3K/protein kinase B pathway may serve as a more reasonable molecular target for meningioma than EGFR 3).