Type of study: Original research (experimental study, engineering approach) First author: Leparc et al. Affiliations:
-
Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
-
Laboratory of Nervous System Disorders and Therapy, GIGA Institute, University of Liège, Liège, Belgium
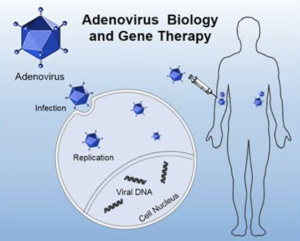
Journal: Molecular Therapy – Oncolytics Purpose: To engineer adenoviruses with modified tropism for systemic delivery—aiming to reduce off-target accumulation and enhance tumor retention via retargeting strategies.
Conclusions: Engineered vectors exhibited improved immune evasion, diminished sequestration by non-tumor tissues, and improved intratumoral delivery, indicating the feasibility of retargeting modifications for systemic adenoviral therapy.
Critical Review
Methodology: The study lacks transparency in the engineering protocol. Crucial elements such as ligand selection, targeting affinity, and vector modifications are insufficiently described. No rigorous dose-responsiveness or replication kinetics are provided.