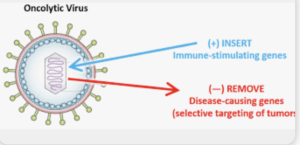
In a Review Raziye Piranlioglu *et al.* from
| Affiliations | Harvey Cushing Neuro‑oncology Laboratories, Dept. Neurosurgery, Brigham and Women’s Hospital, Boston, MA, USA; Dana‑Farber Cancer Institute, Boston, MA, USA |
published in *Seminars in Immunology* with the Purpose to synthesize data from clinical trials of oncolytic viruses (OVs) in glioblastoma, evaluating immunomodulatory effects, delivery strategies, and challenges in assessing immune responses. They concluded that Oncolytic virus therapy is well tolerated in GBM trials and can convert the immunosuppressive microenvironment into an immunologically active state. However, limitations in post‑treatment sampling and delivery methods impede full understanding of biological mechanisms.
This review is a rehash of well‑known take‑home messages, offering little in the way of novel synthesis or incisive critique. The authors lean heavily on canonical trials (e.g., oHSV, adenovirus) but fail to integrate preclinical correlates from myeloid-targeting strategies, such as macrophage polarization dynamics or MDSC modulation. There’s no fresh mechanism, no meta‑analysis of response rates, and no exploration of why most trials remain phase I with limited impact. Sample‑scarcity is once again highlighted as a blocker—but no alternative trial designs (e.g., neoadjuvant window cohorts, liquid biopsy) are proposed. In short, the review scratches the surface of challenges without pushing the field forward.
– Methodology flaws: Reliance on clinical trial summaries without critical assessment of sample sizes, endpoints, or confounders. – Relevance: Glioblastoma is the archetype of immunotherapy resistance—yet this review reiterates, rather than interrogates, that fact. – Novelty: Absent—this is a telescoped summary without new angles on delivery vectors, immune biomarker development, or pivot strategies after failure. – Interpretation errors: The authors imply “immune activation” equates to therapeutic efficacy, with no link to survival or functional endpoints. – Weak conclusions: Acknowledge limitations, but then offer safe, bland suggestions with zero disruptive insight.
Final Verdict
This review is perfunctory—a missed opportunity to provoke meaningful debate or propose technical advances. Strictly descriptive, minimally analytical, and intellectually unambitious.
Takeaway for Neurosurgeons
GBM oncolytic-virus trials confirm feasibility and safety, but therapeutic impact remains marginal, and mechanistic insights are too scant to change practice.
Bottom Line
Safe and well-tolerated, but clinically stagnant.
Score
3 / 10
*Publication date*: June 23, 2025 *Corresponding author email*: rpiranlioglu@bwh.harvard.edu